<strong>Chasing rapid weight loss through keto?
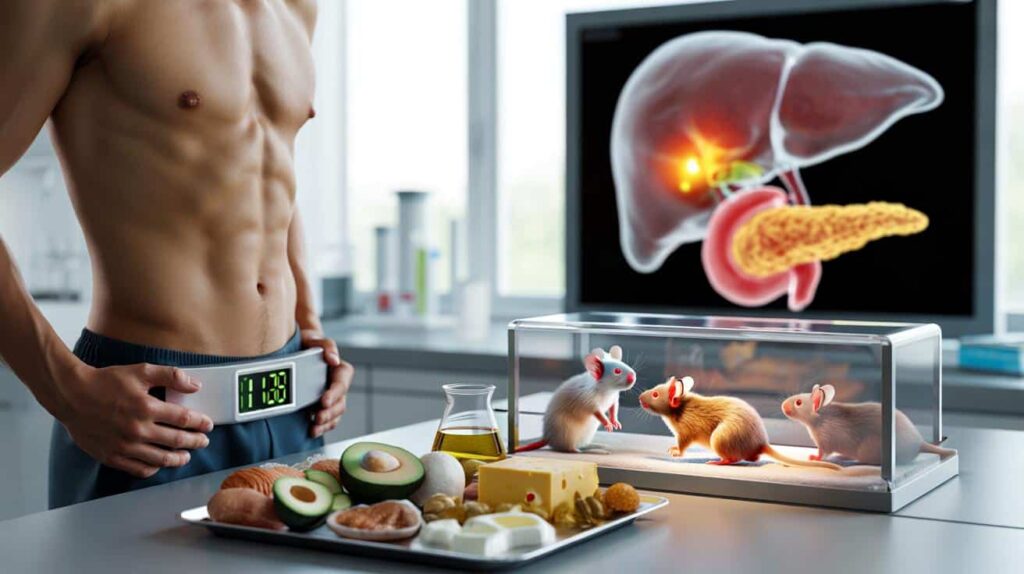
Fresh research in mice hints that trimming the scales might come with an unseen cost.
New findings suggest that the much-hyped ketogenic diet could reshape the body’s metabolism in ways that look far less healthy once you move beyond the bathroom scales.
What this new mouse study actually found
Scientists at the University of Utah followed groups of mice for at least nine months while feeding them different types of diets. One of those diets was designed to mimic a human-style ketogenic plan: very high in fat, very low in carbohydrates, with moderate protein.
The team compared four diets:
- High-fat, Western-style diet
- Very-high-fat, low-carb keto-style diet
- Low-fat, high-carb diet
- Low-fat diet with extra protein to match the keto group
Mice on the keto-like diet did lose less weight than those on the standard high-fat Western diet. At first glance, that sounds like a win for the keto approach. Look closer, and the picture shifts.
Male mice on the keto-style regimen developed fatty liver disease and showed signs that their livers were struggling to function properly.
Both male and female mice on the ketogenic diet also showed disrupted blood sugar control. Their blood glucose and insulin levels dropped within two to three months, but not in a healthy way. Their pancreas cells simply were not producing enough insulin.
Why fat overload is a problem
The core idea behind keto is to push the body into ketosis, a metabolic state where fat rather than sugar becomes the main fuel. That requires slashing carbs and significantly increasing fat intake.
The Utah team’s data suggest that when fat intake is pushed this high, the body’s ability to cope can falter.
As study authors put it, excess fat has to go somewhere, and it tends to accumulate in the blood and in the liver.
In the male mice, those extra lipids built up in liver tissue. Over time, this resembled non-alcoholic fatty liver disease, a condition increasingly seen in people with obesity and type 2 diabetes. The liver helps regulate blood sugar, filter toxins and process fats; when it starts to clog up with fat, the entire metabolic system can drift off course.
Stress on the pancreas and blood sugar control
The story did not stop at the liver. The researchers saw that cells in the pancreas, which normally produce insulin, appeared to be under stress on the keto diet. The animals had low circulating insulin and unstable blood glucose regulation.
That pattern can look deceptively similar to what many dieters aim for: lower insulin and lower blood sugar. In this case, though, the numbers were not a sign of smooth, efficient metabolism. They reflected a system that was not responding properly.
One potential explanation is that a constant flood of fatty acids strains the pancreas, reducing its capacity to release insulin when needed. Over months or years in humans, that sort of disruption might raise the risk of developing type 2 diabetes rather than protecting against it.
Reassuring twist: some changes reversed when keto stopped
When mice were taken off the keto-style diet, their blood sugar regulation improved. Insulin production started to look more normal. That reversibility suggests the body can recover from at least part of the metabolic stress triggered by prolonged ketosis.
The damaging effects seen in mice were not necessarily permanent once the high-fat, very low-carb pattern ended.
Whether the same rebound would happen in humans, and over what timescale, remains unknown. Long-term, carefully controlled human trials are expensive and difficult, and very few have followed people on strict keto plans for more than a year.
What this does and doesn’t say about humans
These results come from mice, not people. Rodents do share many aspects of human metabolism, which is why they are widely used in nutrition and diabetes research, but there are differences in lifespan, diet behaviour and physiology.
So this study does not prove that a human on a ketogenic diet will inevitably develop fatty liver disease or lose proper control of insulin. It does show that the biological mechanisms pushed by keto can have downsides, at least in one mammal species, when maintained long term.
The authors stress the need for more human research that looks beyond weight loss alone. Many short-term studies have shown rapid drops in body weight and blood sugar on keto, especially in people with obesity or prediabetes. Fewer studies track liver health, pancreatic function and overall metabolic stability over several years.
Why keto was invented in the first place
The ketogenic diet is not new. Doctors first used it about a century ago to help children with severe epilepsy when medicines failed. By mimicking the biochemical state of fasting, the diet lowered seizure frequency in many patients.
That medical use continues today, often under close supervision with regular blood tests. In that context, the potential side effects are weighed against the benefit of reducing disabling seizures.
In the past decade, keto has jumped from neurology clinics to Instagram feeds as a rapid-weight-loss and “biohacking” strategy. People attempt their own versions, often relying on online lists of “keto foods” like avocados, eggs, salmon, butter, and nuts, without medical monitoring or lab checks.
The new mouse data suggest that chasing fast weight changes without checking what is happening inside the liver and pancreas could be a risky trade-off.
Practical takeaways for would-be keto dieters
While this study cannot provide direct dietary rules for humans, it helps flag areas to watch. Anyone considering a strict ketogenic plan for months at a time may want to:
- Speak with a healthcare professional, especially if they already have liver issues, diabetes or prediabetes
- Check liver enzymes and blood lipids periodically
- Monitor fasting glucose and HbA1c to track longer-term blood sugar patterns
- Focus on unsaturated fats (olive oil, nuts, oily fish) rather than relying heavily on butter and processed meats
- Avoid excessive alcohol intake, which further stresses the liver
A more moderate approach—such as reducing refined carbohydrates and ultra-processed foods without pushing fat intake to extremes—may deliver many of the everyday benefits people hope to gain from keto, while easing the load on organs that manage fat and sugar.
Key terms worth unpacking
| Term | What it means |
|---|---|
| Ketosis | A metabolic state where the body burns fat and produces ketone bodies as its main fuel instead of glucose. |
| Fatty liver disease | Build-up of fat in liver cells, which can lead to inflammation, scarring and, in severe cases, liver failure. |
| Insulin | A hormone made by the pancreas that allows cells to take up glucose from the blood. |
| Metabolic health | The overall performance of systems that control blood sugar, fats, blood pressure and body weight. |
How this might play out in real life
Imagine two people starting keto at the same time. Both quickly shed several kilos, their waistlines shrink, and blood sugar readings fall. One of them has regular blood tests and finds that liver enzymes are creeping upward and triglycerides are climbing. The other never checks and assumes all is well because the scale is moving in the right direction.
The new mouse research suggests the first person has an early warning that something under the surface is changing. With that information, they could adjust: easing the diet, reintroducing some complex carbohydrates, or switching to a more balanced plan before lasting damage occurs.
Weight loss stories often stop at the number on the scale. Studies like this one remind us that the routes we take to reach that number can leave very different marks on our organs. A diet that looks effective over a season might look less appealing over a decade once the liver and pancreas have had their say.






